Uses of Ammonia The Chemical Man
Ammonia is a compound of nitrogen and hydrogen with the formula NH3. The simplest pnictogen hydride, ammonia is a colourless gas with a characteristic pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers.
Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Ammonia is named for the Ammonians, worshipers of the Egyptian god Amun, who used ammonium chloride in their rituals.
Uses
Fertilizer
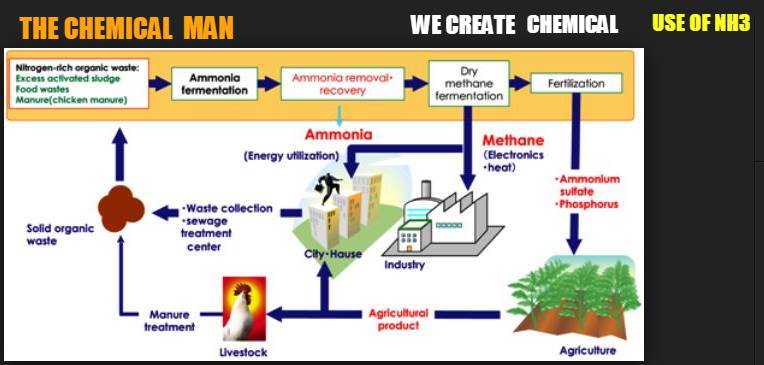
Globally, approximately 88% (as of 2014) of ammonia is used as fertilizers either as its salts, solutions or anhydrously. When applied to soil, it helps provide increased yields of crops such as maize and wheat. 30% of agricultural nitrogen applied in the USA is in the form of anhydrous ammonia and worldwide 110 million tonnes are applied each year.
Precursor to nitrogenous compounds
Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. An important derivative is nitric acid. This key material is generated via the Ostwald process by oxidation of ammonia with air over a platinum catalyst at 700–850 °C (1,292–1,562 °F), ~9 atm. Nitric oxide is an intermediate in this conversion:
NH3 + 2 O2 → HNO3 + H2O
Nitric acid is used for the production of fertilizers, explosives, and many organonitrogen compounds.
Ammonia is also used to make the following compounds:
Hydrazine, in the Olin Raschig process and the peroxide process
Hydrogen cyanide, in the BMA process and the Andrussow process
Hydroxylamine and ammonium carbonate, in the Raschig process
Phenol, in the Raschig–Hooker process
Urea, in the Bosch–Meiser urea process and in Wöhler synthesis
Amino acids, using Strecker amino-acid synthesis
Acrylonitrile, in the Sohio process
Ammonia can also be used to make compounds in reactions which are not specifically named. Examples of such compounds include: ammonium perchlorate, ammonium nitrate, formamide, dinitrogen tetroxide, alprazolam, ethanolamine, ethyl carbamate, hexamethylenetetramine, and ammonium bicarbonate.
Ammonia can also be used to make compounds in reactions which are not specifically named. Examples of such compounds include: ammonium perchlorate, ammonium nitrate, formamide, dinitrogen tetroxide, alprazolam, ethanolamine, ethyl carbamate, hexamethylenetetramine, and ammonium bicarbonate.
As a cleaner
Household ammonia is a solution of NH3 in water (i.e., ammonium hydroxide) used as a general purpose cleaner for many surfaces. Because ammonia results in a relatively streak-free shine, one of its most common uses is to clean glass, porcelain and stainless steel. It is also frequently used for cleaning ovens and soaking items to loosen baked-on grime. Household ammonia ranges in concentration by weight from 5 to 10% ammonia.
Fermentation
Solutions of ammonia ranging from 16% to 25% are used in the fermentation industry as a source of nitrogen for microorganisms and to adjust pH during fermentation.
Textile
Liquid ammonia is used for treatment of cotton materials, giving properties like mercerisation, using alkalis. In particular, it is used for prewashing of wool.
Lifting gas
At standard temperature and pressure, ammonia is less dense than atmosphere and has approximately 45-48% of the lifting power of hydrogen or helium. Ammonia has sometimes been used to fill weather balloons as a lifting gas. Because of its relatively high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard an airship to reduce lift and add ballast (and returned to a gas to add lift and reduce ballast).
Woodworking
Ammonia has been used to darken quartersawn white oak in Arts & Crafts and Mission-style furniture. Ammonia fumes react with the natural tannins in the wood and cause it to change colours.
Energy carrier
Ammonia can be manufactured from solar energy, air and water. This is an efficient way to package hydrogen into a chemical that is much cheaper to store and transport than pure hydrogen be it as gas or as liquid. In fact, per volume ammonia holds more hydrogen than does liquid hydrogen. Ammonia may be the key to overcome not only the daily but also the seasonal fluctuations of renewable energy sources.

Leave a comment